Colorectal cancer (CRC) is the third most common cancer worldwide and the second cause of cancer death.
Chemotherapy and surgery have long been the first line therapies to treat this disease; however, curative treatments are not always achieved, as nearly a quarter of CRCs are diagnosed at an advanced stage with metastases. Furthermore, some of the remaining cases will develop metastasis following the first diagnosis.
While precision oncology for certain cancer types, such as melanoma or breast or lung, has proven to be highly effective, finding clinically-relevant targeted therapies for metastatic colorectal cancer (CRC) has been particularly challenging. This is likely to be due to the genetic heterogeneity observed in CRCs together with the fact that many oncogenic drivers, have been or are still are undruggable.
Nevertheless, as there is a plethora of molecular targets in metastatic colorectal cancer, that have been identified. Below we describe the targeted therapies, their use in the clinic, the limitations they have and the potential strategies to overcome them. We will only focus on those targeted therapies aimed at inhibiting tumour growth, leaving aside the use of immune checkpoint inhibitors for metastatic CRC.
EGFR as a molecular target
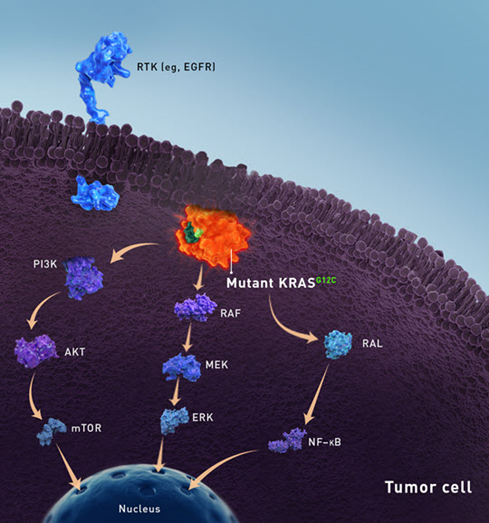
EGFR has been known to be a driver of CRC tumorigenesis, representing an actionable molecular target in various cancers. Its activation leads to various downstream intracellular signalling pathways including the RAS-RAF-MAP kinase (MAPK) pathway and the PI3K-PTEN-Akt pathway, leading to the activation of several transcription factors that control the expression of genes relevant for cell proliferation, cell migration and differentiation.
Overexpression of EGFR has been observed in various cancer types, including CRC. This overexpression is associated with aberrantly high levels of receptor activation as inhibitory mechanisms that control EGFR activation are bypassed.
In 2004, the FDA granted approval for two different anti-EGFR antibodies: cetuximab and panitumumab after they both showed a statistically significant therapeutic effect in patients with chemotherapy refractory metastatic CRC.
Nevertheless, after its clinical implementation, it soon became clear that most of the patients did not respond to this treatment. Subsequent studies demonstrated that up to 40 to 45% of patients with metastatic colorectal cancer harbour mutations on the downstream target KRAS, conferring constitutive activation of MAPK pathway. This explained why these patients were not responsive to EGFR-targeting antibodies.
Currently, the EMA recommends the assessment of KRAS and NRAS status prior to therapy to exclude those patients with mutations. Downstream activation of KRAS signalling also constitutes the main mechanism of acquired resistant to EGFR blockade. Mutations affecting EGFR KRAS, BRAF, NRAS and more rarely MEK frequently emerge following anti-EGFR targeted therapy for metastatic CRC.
Targeting HER2 alterations
Increased levels of HER2 are often observed in patients with advanced -stage cancers or at metastatic stage. HER2 gene amplification, (also known as ERBB2) or mutations within this gene might lead to constitutive activation of the receptor. HER2 homodimerization or heterodimerization with other receptors of the EGFR family results in downstream activation of the RAS-RAF-MAP kinase (MAPK) pathway and the PI3K-PTEN-Akt pathway. Thus, HER2 signalling receptor triggers activation of pro-survival signalling pathways in tumours.
While in other malignancies such as breast cancer or gastric cancer, HER2 amplification occurs at a rate of 25% and 13-22%, respectively, in CRC it occurs at a much lower frequency (within 1-6% of unselected CRC patients). Nevertheless, it is still considered a relevant actionable molecular target in metastatic colorectal cancer.
In addition of being an oncogenic driver, clinical studies suggest that HER2 can also mediate resistance to anti-EGFR antibodies. HER2 amplifications can be targeted by using monoclonal antibodies (such as trastuzumab or pertuzumab) or tyrosine kinase inhibitors (TKIs, namely lapatinib, tucatinib). Clinical trials are been conducted in metastatic CRCs assessing both types of HER2 targeted therapies in various combinations. The objective response rate (ORR) of patients receiving HER2 therapies is around 30%, an encouraging figure considering that these patients have undergone two or more prior regimes. De novo and acquired resistance to HER2 therapies is associated to alterations on RAS or RAF genes. Therefore, patients harbouring mutations in these genes won´t benefit from anti-HER2 therapies.
One of the promising targeted therapies for metastatic colorectal cancer being evaluated involves the use of antibody drug conjugates. In particular, trastuzumab deruxtecan, which links the anti-HER2 antibody to the cytotoxic agent deruxtecan, a topoisomerase I inhibitor has shown compelling activity in the DESTINY-CRC01 trial and has potential to become the first HER2-monotherpay treatment for patients with HER2+ metastatic colorectal cancer.
BRAF-targeted therapies
Around 10-15% patients with CRC harbour mutations within the BRAF gene, and in 90% of the cases represent V600E mutation, leading to constitutive activation of MAPK signalling pathway. In BRAF-V600E mutant melanoma, BRAF kinase inhibitors, such us vemurafenib and dabrafenib are approved with significant overall response rates of 50-80% response rates. Surprisingly, it was observed that only 5% of patients with metastatic BRAF-V600E mutant CRC respond to vemurafenib. The biological explanation for this came from the realization that in CRC no complete inhibition of the MAPK signalling pathway is achieved due to the appearance of an EGFR-dependent feedback causing reactivation of the pathway upon BRAF inhibition. This molecular feedback mechanism is absent in melanomas, as they originate from the neural crest and therefore do not express EGFR. Clinical trials have evaluated the efficacy of BRAF inhibition in combination with anti-EGFR antibodies or inhibitors of downstream molecule MEK in metastatic BRAF-V600E mutant colorectal cancer, leading to the approval of cetuximab (anti-EGFR) and encorafenib (MEK inhibitor) combination for patients with previously treated metastatic BRAF-V600E mutant CRC.
KRAS-G12C inhibitors
In terms of number of patients, KRAS is the most commonly mutated oncogene in human cancer, as it is mutated in cancers with high prevalence, such as lung and colorectal cancer. Numerous failed attempts to target the RAS family of proteins, led to their definition of `undruggable´ proteins. A particular mutation, the G12C variant, has recently emerged as an actionable alteration in KRAS. This variant accounts for up to 44% of all KRAS variants in non-small cell lung cancer (NSCLC) and about 11% in CRC thereby constituting a relevant molecular target in both diseases. The therapeutic agents sotorasib and adagrasib mediates its biological activity by selectively forming a covalent bond with cysteine 12 of mutant KRAS-G12C and locking KRAS in the inactive state. This year, sotorasib was approved for patients with KRAS-G12C NSCLC who have received at least one previous course of treatment. Multiple clinical studies are currently undergoing investigating sotorasib monotherapy or in combination across various advanced solid tumours, including CRC.
The future of targeted therapies
While targeted therapies for metastatic colorectal cancer have been successfully associated with longer survival rates, mechanisms of adaptation and resistance inevitably arise. Resistance is often associated when targeted therapies are administered as monotherapies.

To tackle this, new strategies are being evaluated with various drugs combinations targeting distinct molecular targets within the same signalling pathway. A particular approach consists in using multiple low dose combination of drugs, so that it releases the selective pressure of particular nodes of the pathway, avoiding therefore the selection for drug resistant variants.
Altogether, molecular profiling of individual tumours together with the evaluation of the intrinsic signalling of CRC cells will lead to better outcomes for CRC patients.
Abyntek Research Reagents is our highest quality bioreagents range for cancer research, including more than 6,000 antibodies, proteins and ELISA / CLIA kits for the study of hundreds of biomarkers of different types of cancer.
Discover our Abyntek Research Reagents extensive catalogue and ask for 50% off in your first order here.
References
Greally, Megan, Ciara M Kelly, and Andrea Cercek. 2018. “HER2: An Emerging Target in Colorectal Cancer.” Current problems in cancer 42(6): 560–71.
Lindsay, Colin R, and Fiona H Blackhall. 2019. “Direct Ras G12C Inhibitors: Crossing the Rubicon.” British Journal of Cancer 121(3): 197–98. https://doi.org/10.1038/s41416-019-0499-1.
Di Nicolantonio, Federica et al. 2021. “Precision Oncology in Metastatic Colorectal Cancer – from Biology to Medicine.” Nature reviews. Clinical oncology 18(8): 506–25.
Xie, Yuan-Hong, Ying-Xuan Chen, and Jing-Yuan Fang. 2020. “Comprehensive Review of Targeted Therapy for Colorectal Cancer.” Signal transduction and targeted therapy 5(1): 22.




