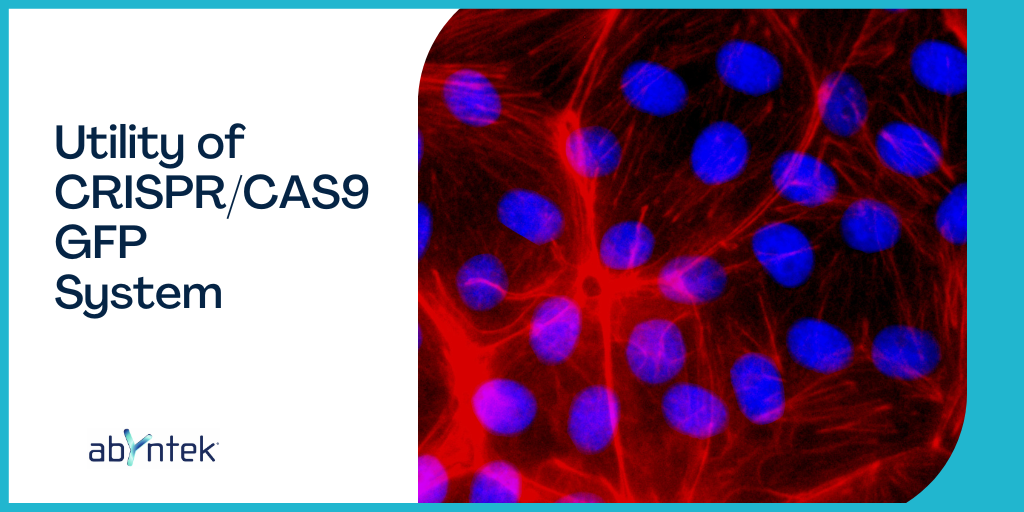
Adding GFP to our experiment gives us the advantage of being able to select cells via fluorescence, separate them if we have a flow cytometer, and also be able to visualize the function of the protein as the cells differentiate and divide.
The utility of CRISPR/CAS9 GFP system for imaging is clear. It is a widely used method to use CRISPR for labelling, gene and protein visualization, as it allows the fluorophore to be integrated into the cell.
In this post, the utility of CRISPR/CAS9 GFP system, the main concepts and a basic protocol are described when carrying out a CRISPR/CAS 9-GFP experiment.
Abyntek provides an extensive catalogue and custom products validated by The Broad Institute of MIT and Harvard ensuring the best quality service. Our experienced scientific team will guide you, boosting your research to another level.

Design and synthesis of CRISPR components
Design of gRNA
- Download the gene sequence and identify the sequence to be impacted.
- Design various sgRNAs. You can use different online tools in order to do it. Tips on how to efficiently design leads in a CRISPR experiment are given in the following posts:
https://www.abyntek.com/how-to-design-guides-for-crispr/?lang=en
https://www.abyntek.com/how-to-avoid-off-target-events-in-crispr-experiments/?lang=en
- Design PCR primers to be able to subsequently detect the sgRNAs sequences and be able to use them as part of the screening.
Donor’s design
When introducing the GFP sequence into the cellular DNA, or also generating a mutation, the DNA donors must be designed. For this, as in the case of the guides, there are programs that ease the task. Sequences for common fluorescent genes are available online. The addition of a fluorescent tag linker peptide to the C- or N- terminal part of the protein is recommended. The sequences for these linkers are also easily found online.
Assembly of the plasmid
Once the vector to be used in the experiment has been selected, a double cutting and ligation reaction must be carried out in the thermocycler, using the restriction enzyme that corresponds to the selected plasmid, the sgRNAs, donors and the enzyme T4 ligase for its subsequent ligation.
Depending of the type of vector, it is possible to directly acquire an all-in-one, which carries all the necessary information for the CRISPR-Cas9 experiment (CAS9 sequence, sgRNA, GFP sequence, sequences corresponding to selection by antibiotics …). Another way, is to introduce each element in a vector. Available commercial vectors normally carry the information on CAS9, GFP in our case, and resistance to antibiotics for the subsequent selection of positive clones.
Vector Production
Once all the information to be inserted into the cells within the vector or vectors is available, it needs to be produced in large quantities. For that purpose, competent cells that subsequently produce the plasmids will be transformed. This blog article discusses how to choose between competent cells:
Subsequently, once the cells produce the plasmids efficiently, they can be extracted from the cells using a plasmid extraction kit. It is convenient to sequence the plasmids before proceeding.
Cell line transfection
The next step is to introduce the vectors inside the cell that we want to modify/mark. We will follow the method related to the type of vector we are using.
Clone Expansion
Clones are expanded by means of cell passages, which are selected thanks to the resistance to antibiotics conferred by the plasmids. Moreover, thanks to the GFP protein, cells that express this protein, modified thanks to CRISPR, can be selected by sorting.
An endonuclease assay can also be performed, which will provide information of the percentage of cells in the population that have been modified.
To continue with the expansion of monoclonal antibodies, serial dilutions are made so that one well will have half the cells of the previous one.
Once the passages to expand the monoclonal antibodies have been completed, the cell genotype has to be verified.
On the one hand, if the cells have been correctly modified, we will be able to measure fluorescence. We must also verify that the information that we have introduced into the cell has modified or not the expression of the gene where it has been integrated. To do this, the first and easiest thing to do is to perform a PCR amplifying the integration site.
The clones that appear as positive must subsequently be sequenced to verify that the integration has occurred correctly.
Abyntek brings CRISPR technology directly to your lab, accompanying you throughout the entire process.
Do you need any help? Contact us filling this form or sending an email to info@abyntek.com. We will be pleased to assist you!