Therapeutic monoclonal antibodies have become the predominant treatment modality for various diseases over the past 25 years.
As a result of the COVID-19 pandemic, several neutralizing antibodies to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have been developed. These monoclonal antibodies bind to the virus in infected patients and are able to ´neutralize´ it. However, it seems that the omicron variant could compromise the effectiveness of the therapeutic antibodies against COVID-19 that are currently in the clinic.
In the following paragraphs we describe what passive immunotherapy consists of and which therapeutic antibodies are effective in fighting the infection with the omicron variant.
Passive immunotherapy
Passive immunotherapy refers to the infusion of antibodies (either monoclonal or polyclonal) to provide protection against infection or envenomation. These antibodies may come from the pooled and purified blood products of immune people or from non-human immune animals.
The earliest antibody-containing therapy used to treat diseases took place in the 19th century and involved diphtheria toxin. Briefly, the blood from animals that had already recovered from the disease was heat inactivated and subsequently injected into guinea pigs. It was observed that animals exposed to these preparations were protected when exposed to lethal doses of diphtheria bacteria or its toxin. After that, scientist showed that they could cure diphtheria in an animal by injecting it with the blood of an immunized animal. This therapeutic strategy was also shown to work in humans and since then passive immunotherapy is used to treat patients after diphtheria or cytomegalovirus infection or as a preventive measure after exposure to a pathogen to try to stop the development of illnesses such as measles, tetanus, hepatitis B, rabies…
The antigen specific monoclonal or polyclonal therapeutic antibodies used today in passive immunotherapies, can be derived from human or non-human blood products:
- Convalescence Plasma Therapy (CPT): refers to the administration of plasma donated by an individual who has had an illness and recovered from it. For instance, people who have been previously infected with SARS-COV2 and have now recovered. Safety risk are minimized by evaluating the presence of infectious agents. Additionally, the titre of the antibody and its neutralization capacity is also measured.
- Humanized mouse that received target antigen: humanized mice are defined as mouse engrafted with functional human tissue. Via genetic engineering, mice have been humanized for their Ig genes to allow for a human antibody response within a mouse background. Thus, this strategy allows to develop fully human therapeutic antibodies which overcome the immunogenicity issues that arise when using monoclonal antibodies that retain mouse sequences. Additionally, recombinant monoclonal antibodies have less limitations than CPT: less risk of transmitting blood diseases, higher titre of antibodies and a particular epitope specificity.
Monoclonal antibodies against COVID-19
While vaccines constitute the best strategy to combat COVID-19 pandemic, certain people that are at high risk of developing severe symptoms could potentially benefit from monoclonal antibodies therapy. Indeed, there is data suggesting that monoclonal antibodies can reduce the risk of COVID-19 hospitalization. Nevertheless, the emergence of variants of concern might confer resistance to some of those monoclonal antibodies.
The omicron variant was first detected in South Africa in November 2021, and it has rapidly spread in many countries across the globe. Its increased transmissibility it is thought to be related to the fact that is heavily mutated: with around 35 mutations in the spike (S) protein and at least 15 mutations in the receptor binding domain (RBD). Because some of these mutations are located within the epitopes of neutralizing antibodies, they might confer immune scape.
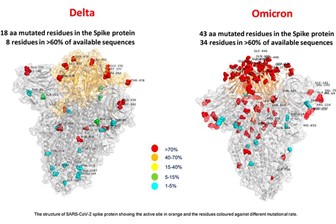
Source: l’Ospedale Bambino Gesù, Roma
The S protein of SARS-CoV-2 plays a key role in virus infection and pathogenesis, and contains the S1 and S2 subdomains, which are responsible for host cell receptor binding and membrane fusion, respectively. In particular the receptor binding domain (RBD) within the S1 subdomain, binds to the human and bat angiotensin-converting enzyme 2 (ACE2) receptors to mediate entry into the cells. Various therapeutic antibodies against COVID-19 which target the S protein are currently in the clinic or in clinical trials. These antibodies bind to the RBD region in the S protein thereby blocking viral entry into host cells. Additionally, these neutralizing antibodies can also exert their therapeutic effect by activating Fc-mediated effector functions, antibody-dependent cellular phagocytosis or antibody-dependent cellular cytotoxicity.
There are several neutralizing antibodies that have been granted approval by the Food and Drug Administration (FDA) and the European Medicines Agency (EMA). However, the extent to which they confer protection from the omicron variant is still under investigation. Below we describe some of the recent updates on the latest research.
The therapeutic antibody sotrovimab neutralizes the omicron variant in vitro
A recent study, which has not been peer-reviewed yet but it is available in MedRxiv preprint server (Cameroni et al. 2021), has evaluated the eight currently approved monoclonal antibodies for their in vitro neutralizing capacity against omicron variant. All, but one of these therapeutics completely lost activity against omicron. The only exception was sotrovimab, showing only a 3-fold reduced neutralizing activity. The study also tested a panel of 36 monoclonal antibodies that are not yet in the clinic, but whose epitope has been characterized structurally. Five of them (S2K146, S2X324, S2N28, S2X259 and S2H97) retained Omicron neutralization, whereas the remaining ones did not.
Sotrovimab was granted Emergency use Authorisation (EUA) by the FDA in late May 2021, and it belongs to the so-called ´super-antibodies´, because of its broad neutralization capacity in the face of emerging variants. This therapeutic antibody, belonging to Vir Biotechnology and GlaxoSmithKline, binds to an epitope on SARS-CoV-2 that is shared with SARS-CoV-1 (the virus that causes SARS), indicating that the epitope is highly conserved, and therefore, resistance might be more difficult to develop. In fact, this antibody was first identified in the blood of a patient who had recovered from SARS and targets an epitope that lies outside of the rapidly evolving RBD region. In contrast, of the other clinically approved therapeutic antibodies target the RBD and therefore do not offer protection against the highly mutated omicron variant.
Additionally, sotrovimab has been designed to contain two-amino acid Fc modifications which increase the half-life of the antibody and potentially improves its bioavailability in the lungs. Of note, based on the clinical trial data, sotrovimab is most effective when taken during the early stages of infection and is indicated for patients with mild-to-moderate COVID-19 who are at high risk for progression to severe coronavirus. The mechanistic insights on how sotrovimab inhibits viral replication are still a matter of investigation, as it does not compete with binding to human ACE2 receptor.
Ongoing studies are also currently evaluating to what extent vaccine-induced neutralizing antibodies offer protection against the omicron variant. Preliminary data suggests that some vaccines do offer some protection, albeit to a lesser extent in comparison to previous variants of concern. Thus, it is likely that booter doses might be needed to achieve effective neutralization titres against omicron.
Do you need omicron-related research reagents? Abyntek can help you. Discover our wide range of antibodies, proteins and ELISA kits for coronavirus research. Contact us for more information.
References:
Cameroni, Elisabetta et al. 2021. “Broadly Neutralizing Antibodies Overcome SARS-CoV-2 Omicron Antigenic Shift.” bioRxiv: 2021.12.12.472269. http://biorxiv.org/content/early/2021/12/14/2021.12.12.472269.abstract.
Dolgin, Elie. 2021. “‘Super-Antibodies’ Could Curb COVID-19 and Help Avert Future Pandemics.” Nature biotechnology 39(7): 783–85.
Taylor, Peter C et al. 2021. “Neutralizing Monoclonal Antibodies for Treatment of COVID-19.” Nature reviews. Immunology 21(6): 382–93.