Flow cytometry is a powerful tool with applications in multiple fields such as cancer biology, immunology, infectious diseases or molecular biology.
It is a technology world-widely used for the study of the immune system´s response to inflammation, pathogens and additionally, cancer. It allows the characterization of mixed cell populations from different sample types, that are tissues, blood, solid tumors or bone marrow.
One of the most important considerations in a flow cytometry experiment is to decide the antibodies to use. At Abyntek Biopharma, we know that flow cytometry is a highly sensitive technique. Thus, we provide exceptional quality antibody custom service for this purpose.
Aplications of Flow cytometry in oncology
Immunophenotyping
Immunophenotyping is the most used application in flow cytometry. It is based on the unique ability of flow cytometry to simultaneously analyze mixed cell populations. Cells need to be stained first with fluorochrome-conjugated antibodies that are targeted against cell surface antigens.
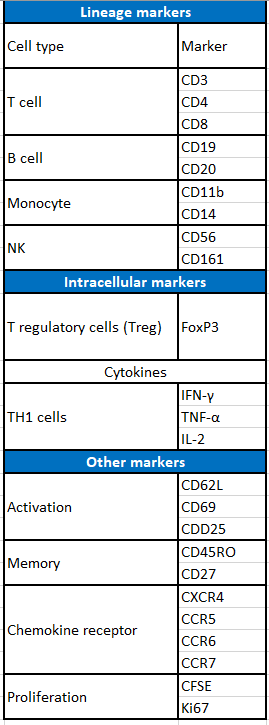
A common nomenclature is used to define these specific monoclonal antibodies, named cluster of differentiation numbers (CD). The vast majority of immune cells have specific CD markers that define them as a population.
Some examples are listed in table 1, and represented in figure 1:
In addition, a live/dead cell dye is commonly use to differs from dead and live cells.
Proliferation and cell cycle analysis
Another use for cytometry is the study of cell proliferation. There are several markers to target proliferation related events, such as incorporation of thymidine analogs (BrdU) into replicating DNA, generational tracking of inheritable permanent dyes (CFSE), and expression of proliferation related antigens (Ki67, PCNA).
The BrdU procedure utilizes DNase to expose the BrdU for antibody stainin. CFSE and other similar dyes (CellTrace Violet, FarRed etc) cross the cellular membrane in living cells and bind covalently and permanently to intracellular structures. Thus, next generation of cells will inherit the dye, allowing for long term proliferation analysis.
The presence of some biomarkers such as Ki67 and PCNA is indicative of cell proliferation. Ki67 is expressed in all cell proliferation phases but not during cell quiescence. Contrary, the proliferating cell nuclear antigen (PCNA) is required for DNA replication.
It is also possible to use flow cytometry to analyze cell cycle. These assays are based on staining DNA with a DNA binding dye.
Apoptosis and regulation analysis
Apoptosis is a phenomenon highly study in immunology and cancer. It is a strongly regulated and controlled process used by the cell to maintain the homeostasis by self-programmed cell dead.
Different events associated with apoptosis can be detected using flow cytometry:
| Event | Staining |
| Plasma membrane translocation | Annexin V |
| endonuclease digestion of DNA | TUNEL assay |
| Caspase activation | Antibody and dye mediated |
| Caspase signaling pathway | Anti-caspase 3 antibody and intracellular dyes |
| Mitochondrial apoptosis | dye |
| Chromatin condensation in the nucleus | Hoescht 33342 fluorescent dye |
Immune check points analysis
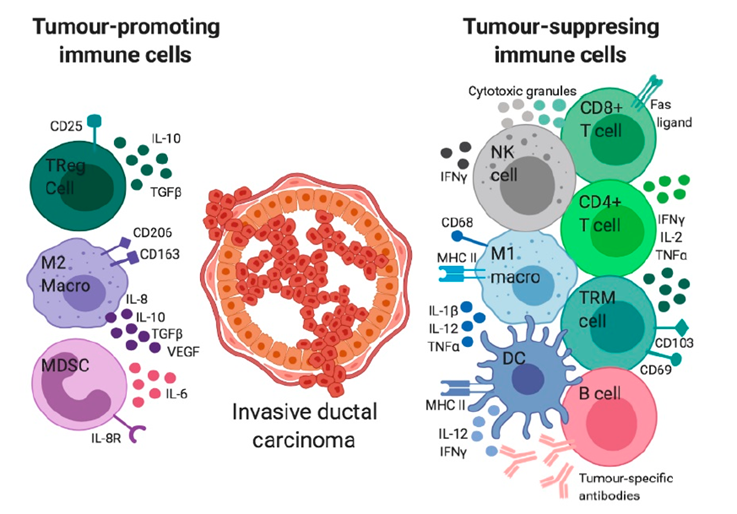
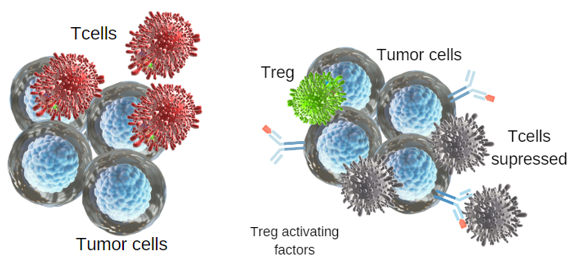
Immune check points are regulators of the immune system. They regulate the immune system’s ability to distinguish itself. Tumor cells use certain immune checkpoint pathways as a method of survival, particularly by inhibiting T cell or NK cell activity. We have already talked about their importance in immune self-tolerance in a previous post.
In a tumor microenvironment activated T cells are recruited to clear tumor cells. However, tumor cells secrete Treg-activating factors. T cell functions are then suppressed by Treg regulatory response. Tumor cells express immune checkpoint repressor PD-L1. Thus, T cells become exhausted by persistent stimulation of expressed PD-1 (Figure 2).
Complementary uses
No matter what kind of cells are the target for flow cytometry, it is possible to use this technique to count the absolute number of cells and also sort them.
For counting the cells, fluorescent beads of a known concentration that is acquired are used along with the sample. The number of beads acquired in the same sample is compared with to the number of cells for the population of interest in the sample.
Furthermore, it is possible to separate cell populations using a flow cytometer with cell sorting capabilities. Cell sorting enables to separate and purify the cells or particles in the sample. Essentially, any cell or particle that can express fluorescence can be separated by a cell sorter. A few types of cells that are commonly sorted are tumor infiltrating lymphocytes, tumor cells, stem cells, transfected cells expressing a fluorescent protein, and white blood cell populations.
May we help you find the antibody you need for your research?
Try our product browser or contact us
Bibliography
Tower, & Ruppert, & Britt,. (2019). The Immune Microenvironment of Breast Cancer Progression. Cancers. 11. 1375. Doi: 10.3390/cancers11091375.
McKinnon KM. Flow Cytometry: An Overview. Curr Protoc Immunol. 2018 Feb 21;120:5.1.1-5.1.11. doi: 10.1002/cpim.40.
Stockmann C, Schadendorf D, Klose R, Helfrich I. The impact of the immune system on tumor: angiogenesis and vascular remodeling. Front Oncol. 2014 Apr 8;4:69. doi: 10.3389/fonc.2014.00069. PMID: 24782982; PMCID: PMC3986554.